Boron in Materials Technology
Attallah, Moataz Mohammad
Mechanical Engineering Department, The American University in Cairo,
113 Sharia Kasr El-Aini, Cairo, Egypt
The first preparation of pure Boron was in 1808 by the French chemists
Joseph Gay-Lussac and Baron Louis Thénard, and separately by the
British chemist Sir Humphry Davy. Since that time, that material has been
divulging a lot of its secrets. Since WW II, the field of Boron chemistry
has significantly grown because of the interesting similarities of Boron
to Silicon and Carbon. Research about its applications has grown rapidly,
especially in the field of using its compounds to produce certain types
of Ceramics. Its compounds have been known for a long time, however, its
applications in materials science and technology did not start except in
the recent years. The boron compounds, notably Boron Carbide and Boron
Nitride, have found for themselves serious applications because of their
outstanding properties, either mechanical, thermal, and physical properties.
Moreover, it has been used to form new types of alloys, particularly with
Aluminum and Titanium.
A-Introduction:
1-Material Profile:
This is the profile of the economic, physical, chemical, mechanical,
thermal, electrical, magnetic and economic properties for Boron: Table
1
Property Value
Boron symbol B
Natural abundance rank in Earth Crust 38
Density 2.35
Atomic Number 5
Mass Number 10.811
Periodic table group III A or 13
Young’s Modulus of Elasticity 400 GPa
Specific Strength 1.40 GPa
Tensile Strength 3.6 GPa
Toughness 17 kJ/ m2
Hardness (Moh’s Scale) +9.300
Hardness (Vickers) 49000 MN/m2
Melting point 2,180°C
Boiling point 3,650°C
Crystalline Boron price 5000$/Kg
Amorphous Boron price 2000$/Kg
2-Metallurgy, ores and preparation:
As mentioned, boron is ranked the 38th in its abundance in earth crust.
The element exists in the following shapes (ores):
a - Mineral Borax=sodium tetraborate: Na2B4O7.10H2O
b - Boric Acid: H3BO3
c - Ulexite: NaCaB5O9.8H2O
d - Colemanite: Ca2B6O11.5H2O
e - Kernite: Na2B4O7.4 H2O
f - Boracite: Mg7Cl2B16O30
Pure boron powder has an amorphous shape (i.e. having no specific shape).
The crystalline pure boron is prepared by dissolving it in molten aluminum
or magnesium and cooling gradually.
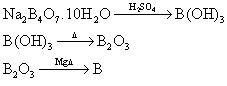 Equations are not balanced; they just show the method of preparing boron.
Equations are not balanced; they just show the method of preparing boron.
Boron can be prepared in the lab, using a good reducing agent:
B2-O3+3Mg -> 3MgO+2B
3-Chemical Aspects:
Despite being in the same group of Aluminum, boron shows non-metallic
chemical and physical properties, similar to carbon and silicon. However,
boron is an electric conductor, like carbon. Thus, boron is considered
a semi-metallic element.
Boron exists in period 2, group 13 (IIIA) of the periodic table,
with valence of 3. The electron configuration of boron is 1s2, 2s2, 2p1.
Boron does not react with water , hydrochloric acid, or hydrofluoric
acid, and it is unaffected by air in room temperature. However, it is reacts
at red hot to form boron oxide (B2O3). Under the same conditions, it reacts
with nitrogen forming boron nitride (BN). With metals, it form metals borides,
such as: magnesium boride (Mg3B2), and Titanium diboride (Ti B2).
Boron has a crystalline Icoshedral appearance(with 20 equilateral
triangles faces, and 12 vertices). Moreover, it has another allotropic
amorphous form.
4-Important compounds:
1-Sodium tetraborate Na2B4O7.10H2O: This
naturally occurring compound has hardness of 2, and specific gravity of
1.7. From its amazing properties that it swells when heated, giving off
water, forming a glass-like material. That is why it is used as a flux
for soldiering and welding because it fuses easily, and dissolves the coating
of metallic oxide, leaving a perfectly clean metal surface . Borax was
used as a disinfectant. However, its most important uses are in the ceramics,
enamel, and glass industry. The famous PyrexTM glass contains 30% borax.
2-Kernite (rasorite): Na2B4O7.4 H2O: This naturally occurring
compound is identical to Borax. Its hardness is 3, and specific gravity
1.95.
3-Boron Oxide : B2O3: This compound is widely used in glass making
because it can withstand high temperatures, and thermal expansion. It is
used in the manufacture of PyrexTM
4-Boric Acid:H3BO3 : This compound is medically used for its
antiseptic properties.
In addition to these compounds, there are two major compounds, which
are: boron carbide, and boron nitride. We specialized a section about each
one, because these two compounds are the most attractive compounds in materials
technology.
2-Boron Carbide:B4C
Boron carbide is a material with excellent properties. The following
table includes a profile for that material:
Property Value or description
Specific gravity 2.51
Crystal structure Rhombohedral
Hardness +9.50(Moh’s scale)
Melting point 2450°C
Poisson’s ration 0.21
Young’s Modulus 480 GPa
Ductility 0.48 %
Other unlisted properties include: low thermal conductivity,
results in poor thermal shock resistance.
Boron carbide is prepared by the reaction between boric acid or boron
oxide with carbon containing compounds in electric arc furnaces.
2B-2O3+7CàB4C+6CO
That method is called hot pressing or cold forming and sintering. This
method results in a dense, self-bonded body, retaining the properties of
a single crystal material.
With all the previously mentioned properties, boron carbide
has emerged lately in materials technology as an excellent substitute for
other strengthening agents, such as: silicon carbide, for applications
that requires high wear resistance and high stiffness. Nowadays, boron
carbide is used as a reinforcing element for aluminum and titanium.
The applications if boron are listed as follows:
1 - Wear resistant components.
2 - Armor tiles in military purposes; it was first used
in Vietnam war as a light hard bullet proof armor for helicopters and tanks.
It is used also as armor for vital equipment. For example, it was used
by NASA to protect the shield of the space shuttles.
3 - Abrasives: lapping and polishing powders.
4 - Raw material: in preparing other boron compounds,
notably titanium diboride: 2TiO2+B4Cà2TiB2+4CO
Directory
Home